In BCR-ABL1 positive leukemia, mutations in the BCR-ABL1 kinase domain (KD) are the most common TKIs resistance mechanism. Sanger sequencing (SS) is currently the gold standard for detecting ABL1 KD mutations, but its low detection sensitivity cannot reveal mutations below 20% VAFs (variant allele frequency), and it is challenging to distinguish compound or polyclonal mutations. In recent years, the NGS-based BCR-ABL1 KD mutation screening protocol provides a powerful tool for deciphering complex patterns (compound or polyclonal mutations) and higher detection sensitivity (~2%, hotspot mutation 1%).
Based on an inhouse designed NGS-based BCR-ABL1 KD mutation screening protocol, we retrospectively analyzed 348 specimens that undergone SS (ALL, n=164, CML, n=128, CML-BP, n=33, AML, n=3, other, n=20, other includes MAL, B-LBL). The BCR-ABL1 transcripts of 109 cases were 0, 184 cases were >0.1%, and the remaining 55 cases were between 0-0.1%. NGS screening results showed that the average VAF was 32.81% (range, 1.0%-100%), which was not provided by SS.
Compared with SS, NGS screening provided a higher mutation-positive rate (22.13% vs. 33.05%, P =0.0013), and Gatekeeper T315I remained the most common BCR-ABL1 KD mutation (12.24% vs. 14.06%, P =0.4513). When the BCR-ABL1 transcript was 0, no mutation was detected in SS; while NGS ABL1-KD discovered mutations in 12 cases (0 vs. 11.01%, P =0.0004) with VAFs lower than 10% except for one S438F (VAF 14.9%) mutation,among them are the well-known F359V and E450G, as well as ten rare mutations, including T277A and E494K.
There were also significant differences in mutations between 0 and 0.1% in BCR-ABL1 transcripts (16.36% vs 32.73%, P =0.046). However, when the BCR-ABL1 transcript >0.1%, there was no difference in mutation-positive rate between the two groups (36.96% vs. 46.20%, P=0.072).
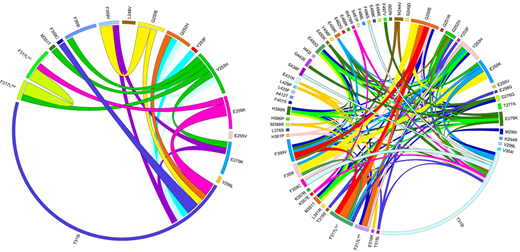
NGS screening disclosed more multiple mutations (59 vs 82, P =0.03), with significant advantages in detecting >3 mutations (3 vs 18, P = 0.0009). Compound mutations (CMs) were determined in 8 cases (8/384, 2.08%), with the incidence of 24.24% (8/33) in cases which carrying two or more mutations, of which T315I-including CMs were the most common (6/8, 75%).
CMs or polyclonal mutation analysis is particularly crucial for severe patients who have received multiple consecutive TKIs treatments, and those with multiple background mutations and a higher risk of progression to clinical resistance. Our results showed that the inhouse designed NGS-based screening protocols could decipher TKIs resistant mutations more comprehensively than SS(Fig 1), and worthy of being implicated in clinical practice.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal